Chapter 1: The Fundamentals of Chemical Biology (第 1 章 化学生物学基础)
1.0 INTRODUCTION (引子)
1.0.1 Why organize a book on chemical biology around biooligomers? (为什么要围绕生物大分子来编写一本关于化学生物学的书)
In a view of subject concept:
- Chemistry offers insight into the behavior of molecules. (For its subdiscipline, doing so with a limited range)
- Organic chemistry compromises between qualitative and quantitative approach, capable of explaining the molecular diversity for evolution of organisms.
- Chemical biology applies the rules of chemistry to biological systems.
从学科思想的角度来看:
- 化学是研究分子行为的学科。(化学的分支学科则在特定范围内研究)
- 有机化学平衡定性与定量方法,可用于解释生物进化所需的分子多样性。
- 化学生物学将化学规则应用于生物系统。
p.s. "Compromise" here manifests a certain degree of equilibrium, which contains both strict quantum chemistry and empirical laws, for me. Later it would be expanded in Chapter 2.
注:我认为这里原文的“compromise”体现了有机化学在严谨的量子化学和经验规律之间的平衡(译文“一种特有的定性和定量方法”翻译的并不甚好),本书第二章对此有具体说明。
Combinatorial assembly strategy
- means to modularly assemble a diverse population from limited subunits.
- could be appreciated in the "letter => word => sentence => paragraph" organization of writing.
- generates diverse populations at different levels of living system.
组合策略
- 指以有限单元模块化组装成多样高级单元的过程。
- 可在“字母 => 单词 => 句子 => 段落”的英文写作组织中体现。
- 在生命系统的不同层次产生多样化的群体。
The central dogma of molecular biology
- governs the assembly of biooligomers.
- is the thematic organization for this book.
分子生物学的中心法则
- 控制着生物分子的组装。
- 是本书编写的主线。
p.s. Back to my high school days, my biology teacher highlighted central dogma as the flow of genetic information. Here, the diversity behind evolution weighs strongly with the authors, for which the importance of "flow" appears as the government of biooligomer assembly. (The author also used "biooligomer" rather than common "biomolecule" to underscore the significance of "assembly")
注:我高中的生物老师就常常强调中心法则表明了遗传信息的流向。本书的作者把驱动生物进化的多样性作为化学生物学核心思想之一,在这种认识上中心法则的流向也是生物分子组装的决定方向。我们同时也注意到本书作者表达“生物分子”常用的是“biooligomer”一词(直译为“生物聚合物”),其强调“组装”之意不言自明。
1.2 GENES (基因)
1.2.1 A gene is made up of a promoter and a transcribed sequence (基因由启动子和转录序列组成)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
A gene
- contains a transcription-factors-binding promoter and a RNA-encoding DNA sequence.
- is often organized into an operon or cluster of genes with other genes which are functionally related and controlled by the same promoter. (McC operon in E. coli as an example)
一个基因
- 包含能结合转录因子的启动子序列和能编码 RNA 的一段 DNA 序列。
- 常常和其它功能相关的基因被整合在同一个操纵子(或基因簇)中,这些基因具有相同的启动子。(如大肠杆菌中的 McC 操纵子)
p.s. Latent translation mistake: the original text says "the control of a common DNA promoter sequence", where "common" obviously means "shared" rather than "usual".
注:此处可能有翻译错误,原文说“the control of a common DNA promoter sequence”。这个“common”显然指的是“共同的”,而非“常见的”。

Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 4, Figure 1.6
The smallest gene makes a chemical weapon
(A) Microcin A is encoded by the tiny mccA gene grouped together with related genes in the McC operon. These genes are made up of DNA. (B) The peptide microcin A is assembled into a sleek chemical weapon. Me is a commonly used abbreviation for a methyl group (CH3).
1.3 GENOMES (基因组)
1.3.1 We have sequenced the human genome and many others. Now what? (我们已经对人类基因组和许多其他基因组进行了测序,现在该做什么?)
The gap between sequencing protein and understanding its function is from
- the difficulty for studying related non-protein biooligomers.
- the lack of certain conditions for protein of interest to function in experiment.
- the lack of complete dynamic system in experiment.
了解蛋白质序列和理解蛋白质功能相差甚远,这是因为
- 许多蛋白质的功能和不易研究的生物分子有关。
- 许多蛋白质只能在一些特定条件下发挥相关功能。
- 许多蛋白质只有在动态的整体中才能发挥功能。
1.3.2 We are far from understanding cells that we understand the best—Escherichia coli (我们还远不了解我们认为最了解的大肠杆菌)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
E. coli
- has numerous strains, with the common laboratory strain being E. coli K-12.
- is typically a little more than 1 μm in diameter.
- has a complex coating composed of two fluid lipid membranes and a sandwiched tough, web-like cell wall in the periplasm.
- could fission into cooperative colonies with chemical signaling under nutrient-rich conditions.
- could exchange genetic material with other strains with thin hair-like projections on its surface.
大肠杆菌
- 存在多种菌株,其中实验室常见的是 K-12 菌株。
- 直径通常略大于 1 μm。
- 的外壁是双层膜结构,坚韧网状的细胞壁存在于两层膜间的周质间隙中。
- 在营养丰富的条件下可以分裂形成生产合作的菌落,菌落内部存在着化学信号。
- 可以通过表面的发状突起和其他菌株交换遗传物质。
p.s. The mentioned "hair-like projections" might be the conjugative pilus. A illustration is attached below for explantion.
注:这里说的“发状突起”指的可能是接合菌毛,下方的插图图注中有进一步说明。

Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 6, Figure 1.10
Inside a bacterium
Diagram of E. coli K-12 showing the arrangement of membranes and the location of genomic DNA and ribosomes. E. coli K-12 was originally isolated from the stool specimen of a diphtheria patient in 1922.
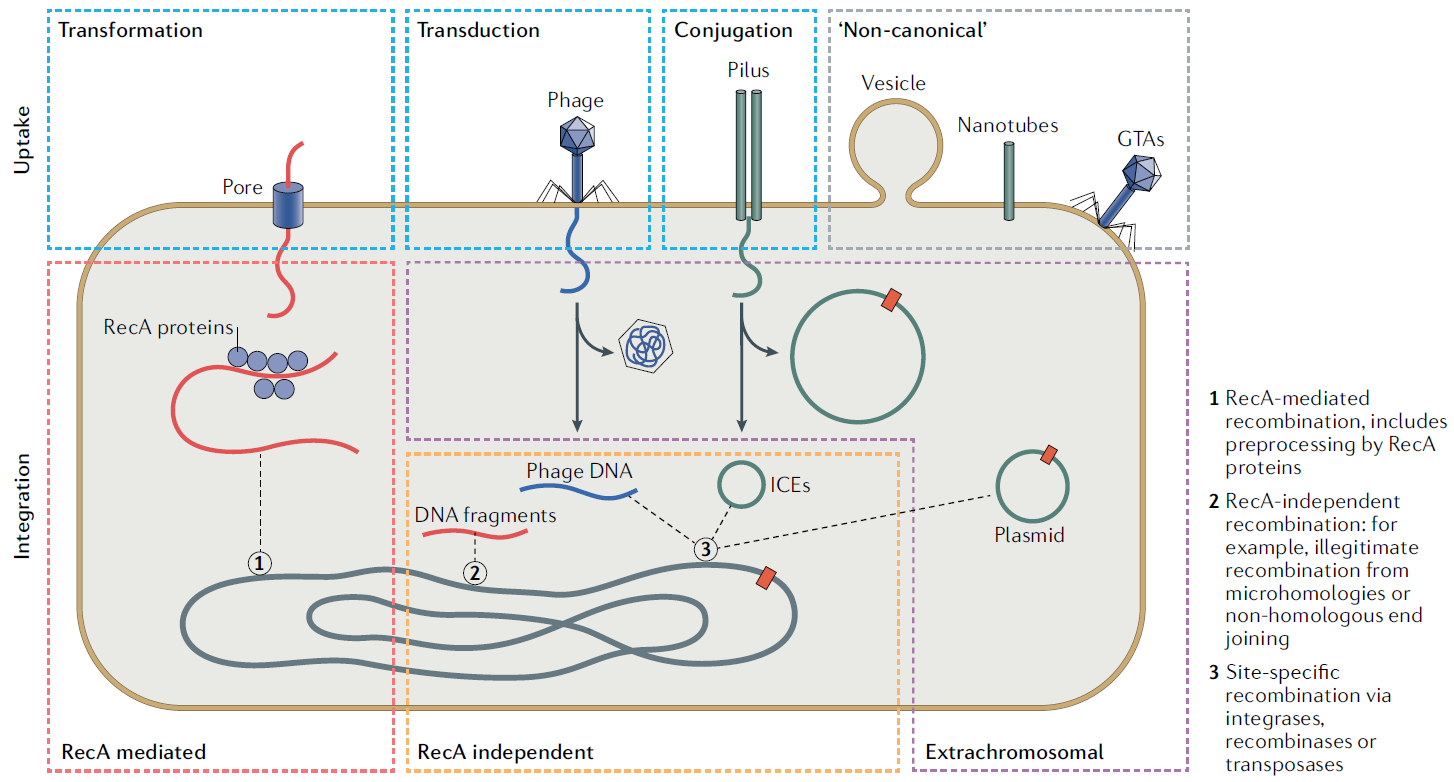
Nat. Rev. Microbiol., 2022, 22, 206-218, Figure 1
Overview of mechanisms of DNA uptake and integration (only relevant captions are attached)
... Conjugation often transfers plasmids and other conjugative elements by direct contact via a conjugative pilus...
1.3.4 You cannot judge a cell by its genome (我们不能仅通过基因组来判定一个细胞)
In a view of genome similarity:
- Strains of E. coli have very different genomes.
- Mammals (such as mice and humans) share 99% of the same genes.
- All humans of the same sex are 99.9% identical.
从基因组相似度的角度看:
- 大肠杆菌不同菌株基因组差异很大。
- 哺乳动物(如老鼠和人类)中同源基因的比例高达 99%。
- 同性别人类的基因组 99.9% 是相同的。
p.s. Latent translation mistake: The original text goes that "Strains of E. coli have similar structures but very different genomes, whereas the opposite is true for mammals such as mice and humans, which share 99% of the same genes." It is hard to tell the exact meaning of "the opposite", is it an intraspecie sequence similarity or an interspecie gene homology? A reliable clear description from Lewin's Gene Ⅻ says "human and chimpanzee genomes shares 98.5% sequence similarity", which makes me correct the translation into gene homology.
注:此处可能有翻译错误,原文说“Strains of E. coli have similar structures but very different genomes, whereas the opposite is true for mammals such as mice and humans, which share 99% of the same genes.”。这里并不好直接读出“99%”指的是种类序列相似性还是种间基因同源性。我参看了《基因Ⅻ》,其中提到人类和黑猩猩的序列相似度就能达到 98.5%,我因而认为 99%值的更可能是基因同源性。
1.4 SOURCES OF DIVERSITY BEYOND GENOMES (基因组以外的生物多样性来源)
1.4.2 RNA splicing amplifies the diversity of the transcriptome (RNA 剪接增加了转录组的多样性)
Splicing
- cannot be predicted from DNA sequence.
- depends on environmental conditions.
剪接
- 位点仍无法由 DNA 序列预测得到。
- 同时取决于环境条件。
1.4.3 Post-translational modification of proteins amplifies the diversity of the proteome (蛋白质的翻译后修饰增加了蛋白质组的多样性)
Post-translational modifications include:
- trimming
- splicing
- phosphorylation
- glycosylation
- oxidation
- addition of membrane anchors
- fusion with other proteins
- alkylation
- acetylation, etc.
蛋白质翻译后修饰包括:
- 修剪
- 剪接
- 磷酸化
- 糖基化
- 氧化
- 添加膜锚定标签
- 与其他蛋白融合
- 烷基化
- 乙酰化等
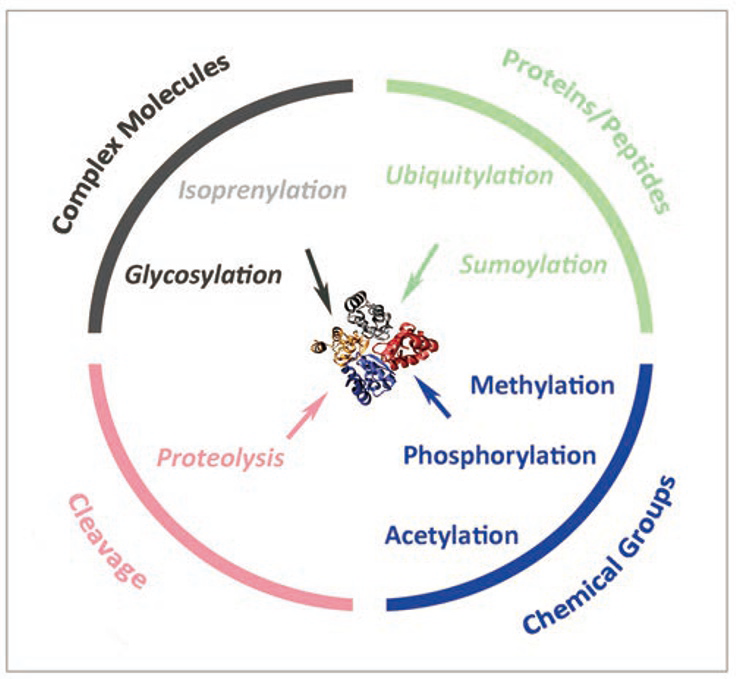
Cell Res., 2014, 24, 143-160, Figure 1
(only relevant captions are attached)
Proteins in eukaryotic cells can be edited after translation by a wide variety of reversible and irreversible PTM mechanisms. The structure, stability and function of proteins in the cells can be dynamically altered by these PTMs...
1.4.4 Beyond template-directed synthesis of biooligomers (非模板导向的生物分子的合成)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
Enzymes synthesize some biooligomers without biooligomer template,
- which are polyketides, oligosaccharides, and terpenes.
- where the enzyme-encoding genes are mostly randomly ordered in genome, with exceptions of some important polyketide synthases.
有些生物分子的酶催化合成不需要以其他生物分子作为模板,
- 如聚酮、糖类和萜类化合物。
- 编码有关酶的基因在基因组中常常任意排列,一些重要的聚酮合成酶基因除外。
Further modification could be done on polyketides, oligosaccharides, and terpenes,
- such as cyclization, oxidation, reduction, and cleavage.
- which may mask the initial structure.
聚酮、糖类和萜类化合物在合成后也可被进一步修饰,
- 如环化、氧化、还原和裂解。
- 这些修饰会使最终产物的结构大不相同。
1.5 COMBINATORIAL ASSEMBLY GENERATES DIVERSITY (组装产生的多样性)
1.5.4 The human immune system uses combinatorial biosynthesis (人类免疫系统使用组合生物合成策略)
The diversity of antibody is from
- varible (V)-diversity (D)-joining (J) module library combination for light chain-heavy chain pair.
- imprecise joining of genetic modules.
- hypermutation in V, D and J modules in B lymphocytes.
抗体的多样性源于
- 以可变区,差异区和连接区模块文库的组合策略产生轻重链组合。
- 以不精确的方式连接基因模块。
- B 淋巴细胞中 V、D、J 区的高突变频率。
1.6 SOME COMMON TOOLS OF CHEMICAL BIOLOGY (一些常见的化学生物学工具)
1.6.3 Powerful microbiological screens reveal interesting chemical phenomena (强大的微生物筛选揭示了有趣的化学现象)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
Bacterial selection
- is ideal for assay of extremely high throughput.
- may fail due to the resilience of bacteria population from natural mutation.
细菌选择
- 可以应用于极高通量分析场景。
- 可能因为细菌种群的自然突变而无效。
1.6.4 Viruses deliver genes efficiently (病毒高效地传递基因)
Importance of viruses in molecular biology:
- Viral gene delivery is the only non-disruptive method for gene delivering.
- In order to overwhelm the host enzymes, viral enzymes are commonly superior in efficiency.
- Viruses are lean and simple.
病毒在分子生物学中的重要性:
- 病毒是唯一的非破坏性基因递送方法。
- 为了“接管细胞”,病毒编码的酶往往更加高效并适合作为工具酶。
- 病毒既精密又简单。
1.6.5 Vast libraries of proteins can be screened in vitro using bacteriophages (可以使用噬菌体在体外筛选大量的蛋白质文库)
Phage display technique
- used engineered recognition protein on phage surface to screen ligand-binding protein.
- uses phage infection to re-establish a population of clones.
噬菌体展示技术
- 利用在噬菌体表面展示目标蛋白进行分子识别蛋白的筛选。
- 利用噬菌体感染细菌来重建克隆群体。
p.s. Latent translation mistake: The original text says "for the ability to cling to a surface" rather than "cling to a protein".
注:此处可能有翻译错误,原文说“for the ability to cling to a surface”,而非“cling to a protein”。翻译成“对目标蛋白的结合能力”并不合适。
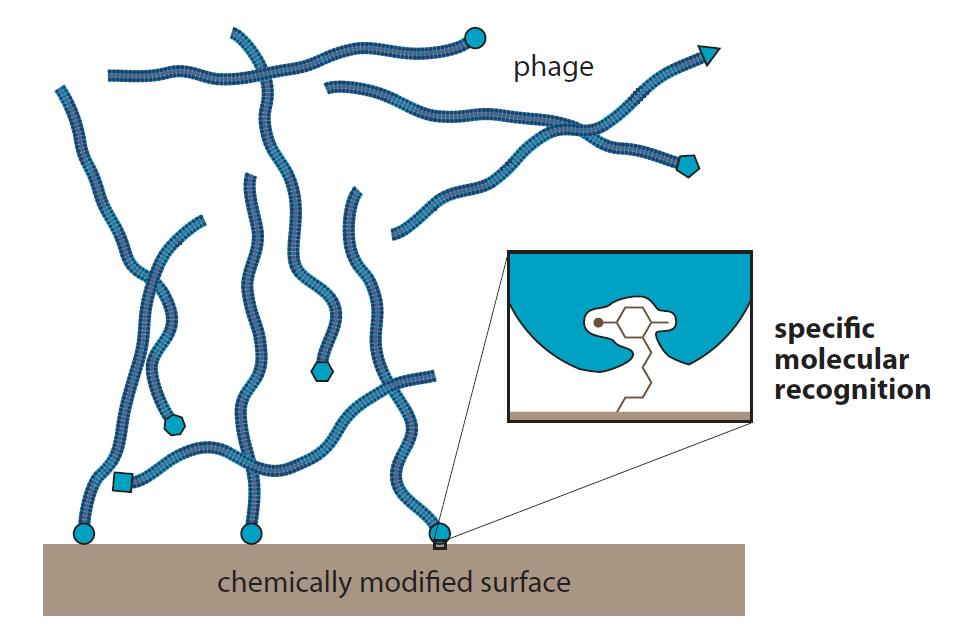
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 19, Figure 1.34
Virus libraries
Libraries of millions of filamentous phage, eachwith a different protein at the tip, can be screened for the ability to recognize molecules immobilized on a surface.
1.6.8 Short RNA molecules silence gene expression (短链 RNA 分子沉默基因表达)
Advantages of RNA interference:
- Short synthetic RNA can enter human cells.
- Design and synthesis of short RNA are much easier than those of small molecules.
RNA 干扰技术的优势:
- 短链 RNA 分子可以穿过细胞膜进入人类细胞。
- 相较于小分子,短链 DNA 的设计和合成更为便捷。
Obstacles for RNA interference:
- The process of RNA cell entry in poorly understood. (Injection delivery fails for non-eye organs)
- Potential off-target effect.
- RNA interference can only affect protein translation rather than translated protein.
RNA 干扰技术发展的制约:
- 人们对 RNA 进入细胞的过程知之甚少。(注射递送仅对眼睛有效)
- 潜在的脱靶效应不易评估。
- RNA 干扰只能阻止蛋白质产生,而无法影响已经成熟的蛋白质。
1.6.11 Model organisms teach us about humans (模式生物教给我们关于人类的知识)
p.s. Latent translation mistake: A more well-acknowledged translation of "model organism" exists.
注:此处可能有翻译错误,“model organism”更常见的翻译显然是“模式生物”而非“生物模型”。
Why we study microscopic soil worms and fruit flies?
- As a consequence of evolution, worms, flies, and humans share similar genes, similar proteins, and similar biochemical mechanisms.
- C. elegans is transparent, allowing one to see the cells and the organs in developmental biology study.
- D. melanogaster has more human-like stages of embryological development.
为什么我们要研究土壤蠕虫和果蝇:
- 从进化的角度看,蠕虫、苍蝇和人类共享相似的基因、蛋白质和生化机制。
- 秀丽隐杆线虫是透明的,这使得发育生物学家可以直接观察其内部的细胞和器官。
- 黑腹果蝇胚胎发展的阶段更接近人类。
Chapter 2: The Chemical Origins of Biology (第 2 章 生物体的化学起源)
2.0 INTRODUCTION (引子)
2.0.0 Introduction (引子)
Erwin Schrödinger's arguement in What is life:
- All living things are governed by the same physical laws as those encountered in everyday life.
Erwin Schrödinger 在《生命是什么》中提出重要论断:
- 所有生物体系都遵循着与我们日常生活中所遇到的相同的物理学定律。
2.1 MECHANISTIC ARROW-PUSHING IS AN EXPRESSION OF MOLECULAR ORBITAL THEORY (电子转移机理是分子轨道理论的一种表达形式)
2.1.1 Three properties control chemical reactivity (三大性质控制化学反应性)
Arrow-pushing
- was first introduced by Robert Robinson and Arthur Lapworth in 1922. (Before quantum mechanics)
- needs us to choose curved arrow sets according to the energy of transition state.
箭推机理
- 由 Robert Robinson 和 Arthur Lapworth 在 1922 年首次使用。
- 的书写主要取决于对应历程的过渡态能量。
p.s. Here, I choose to translate it by myself to convey both "arrow in form" and "mechanism behind". To be honest, the original narrative is quite interesting but personal. A better logic chain would be "using arrowing-pushing to present mechanism and choosing mechanism by its transition state energy".
注:这里我尝试自己翻译了一下,我认为“箭推机理”这个表述既照顾了箭头的形式又包括了机理的内涵。其实作者在这个部分的叙述是较为个人化的,尽管十分有趣,但我认为更好的说法可能是“依据过渡态能量判断一种机理的可行性,并用箭头恰当的表现”。
A basic three-term equation to quantify the energy of transition state could be
- \(\mathrm{Energy} \quad \propto \mathrm{Charge \, Interactions} \quad + \mathrm{(Non \mbox{-} charge) \, Repulsive \, Interactions} \quad - \mathrm{(Non \mbox{-} charge) \, Attractive \, Interactions}\)
反应过渡态的能量可以分为三部分,由下式进行估算
- \(\mathrm{能量} \quad \propto \mathrm{电荷相互作用} \quad + \mathrm{(非电荷)排斥作用} \quad - \mathrm{(非电荷)吸引作用}\)
p.s. I was pretty perplexed the first time I saw this equation and had two question: 1) When we are talking about charge interaction it could be repulsive or attractive, so why "repulsive" again in the second term? 2) Let's say the last two terms are "non-charge interaction", then the equation is quite a rubbish. However, it worth to be presented in this way regarding later expansion and I add "non-charge" here. By the way, the mathematical signs here are obscure as well. It might means "Basing on the consideration of charge interactions, non-charge repulsive interactions increase it and non-charge attractive interactions decrease it".
注:其实我第一次看这部分的时候完全没有理解,当时产生了两个疑问:其一是电荷作用有正有负,为什么后两项还在说“吸引”和“排斥”;其二是就算后两项是默认非电荷作用,那这个式子也是废话一句。当然,我看到下文的扩展后才理解了作者这样写的意图。此外,作者这里用了十分晦涩的数学符号,我认为他可能是想表达“我们首先可以基于电荷相互作用得到一个过渡态能量,在此基础上,额外的非电荷排斥作用使能量升高,而非电荷吸引作用使能量降低”这样一个意思。
2.1.2 Perturbational molecular orbital theory connects arrow-pushing with quantum mechanics (微扰分子轨道理论将箭推机理与量子力学联系在一起)
Ian Fleming developed the equation vide supra into a qualitative expression of perturbational molecular orbital theory
- \(\mathrm{Reaction \, Energy} \quad \propto \mathrm{Coulomb's \, Law} \quad + \mathrm{Sterics} \quad - \mathrm{Filled \mbox{-} unfilled \, Orbital \, Overlap}\)
Ian Fleming 通过定性表达微扰分子轨道理论发展了对过渡态能量的比较方法
- \(\mathrm{反应能量} \quad \propto \mathrm{库仑定律} \quad + \mathrm{空间效应} \quad - \mathrm{填充轨道与未填充轨道的重叠}\)
About the equation of I. Fleming:
- Sterics is actually from the overlap of filled orbitals.
- Filled-unfilled orbital overlap alone is the underpinning of arrow-pushing.
关于 I. Fleming 的扩展式
- 空间效应产生于填充轨道之间的相互排斥。
- 填充轨道与非填充轨道的重叠才是箭推机理的根本。
2.1.3 Six canonical frontier orbitals can be used to predict reactivity (六大经典分子轨道用来预测化学反应性)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
p.s. Latent translation mistake: "canonical" here could be regarded as a advance "classical".
注:此处可能有翻译错误,这里“canonical”这个词指的其实就是“经典的”之意。
In a view of (hybrid) atomic orbitals:
- p orbital has higher energy than s orbital.
- sp3 hybrid orbital has higher p character and higher energy.
- Basicity of carbanions could be explained in this way.
从(杂化)原子轨道的角度看:
- p 轨道相比 s 轨道能量更高。
- sp3 杂化轨道的 p 成分更高,因此能量更高。
- 我们可以解释不同碳负离子的碱性差别。
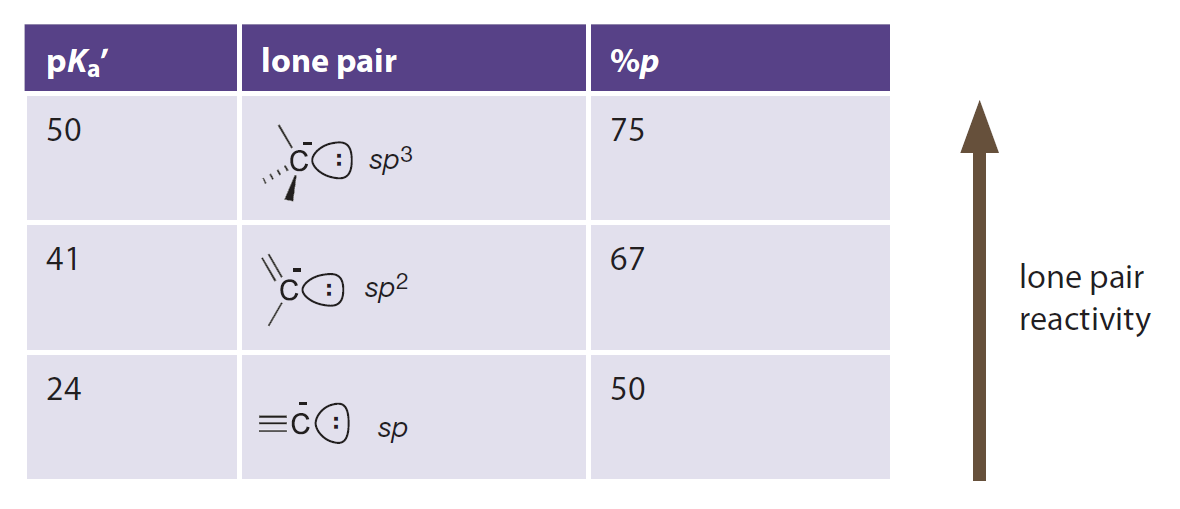
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 29, Table 2.1
p Character and basicity
In a view of frontier molecular orbitals:
- Six canonical types of frontier molecular orbitals in order of energy from low to high are: σ, π, nonbonded lone pairs (n), empty p orbitals, π* and σ*.
- Energy order of filled frontier orbitals could be explanation of nucleophilicity.
- Energy order of unfilled frontier orbitals could be explanation of electrophilicity.
从前线分子轨道的角度看:
- 六种经典前线分子轨道按照能量从低到高排序为:σ、π、非键孤对电子 n、p 空轨道, π* 和 σ*。
- 填充的前线分子轨道的能量顺序可以用于解释物种亲核性。
- 未填充的前线分子轨道的能量顺序可以用于解释物种亲电性。
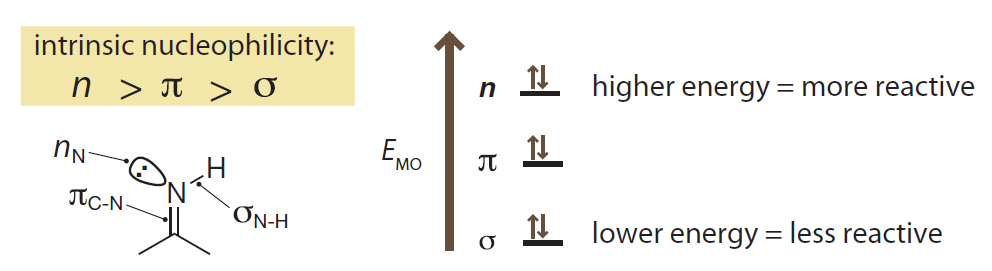
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 30, Figure 2.7
Filled frontier orbitals
The relative energies of the three types of canonical filled orbitals are a good predictor of the nucleophilicity of the electrons that occupy them.
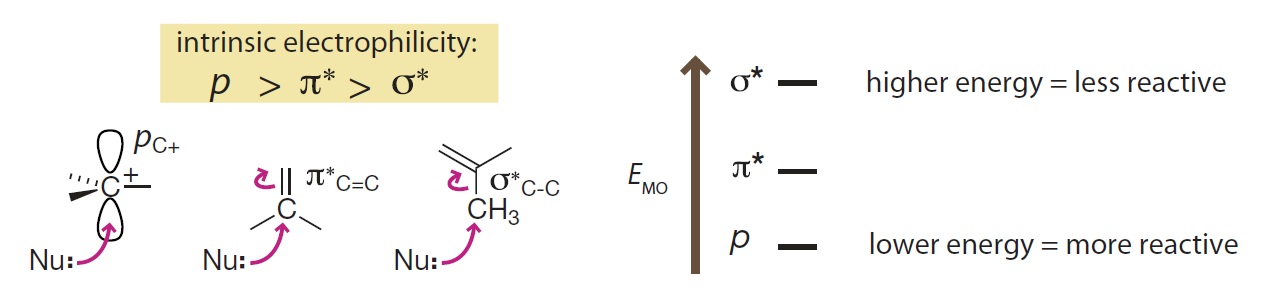
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 31, Figure 2.8
Unfilled frontier orbitals
The relative energies of the three types of canonical unfilled frontier orbitals are a good predictor of electrophilicity.
2.1.4 Electronegativity affects both frontier orbitals and Coulombic interactions (电负性影响了前线轨道和库仑相互作用)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
Electronegativity affects reactivity in a complex manner:
- Atom of high electronegativity lowers the energy of all orbitals.
- Partial charge from electronegativity also affects Coulombic interactions.
电负性对反应活性有着双重影响:
- 高电负性的原子将会使所有轨道的能量降低。
- 电负性通过影响部分电荷影响库仑相互作用。
Two broad generalizations will cover most of the biochemical reactions:
- Focus on the decrease of orbital energy when a nucleophile attacks on carbon atoms.
- Focus on the increase of negative charge when a nucleophile attacks on cations.
对于大多数生化反应可以参照以下两条普适性结论进行判断:
- 对亲核试剂进攻碳原子的反应,我们应该关注高电负性原子的前线轨道能量更低。
- 对亲核试剂进攻质子或正离子的反应,我们应该关注高电负性原子携带更多的负电荷这一事实。
2.1.5 Curved mechanistic arrows depict the interaction of filled orbitals with unfilled orbitals (弯曲的反应机理箭头描绘填充轨道与未填充轨道的相互作用)
Arrow-pushing mechanisms
- are more art than science.
箭推机理的书写
- 更像是一门艺术而非科学。
Curly arrows
- according to I. Fleming, "illustrate the electron distribution in the frontier orbital".
- depict the interaction of filled orbitals with unfilled orbitals.
弯曲箭头
- 在 I. Fleming 看来“描绘了前线轨道的电子分布”。
- 描述的是填充轨道和未填充轨道的相互作用。
2.1.6 There are three basic rules for mechanistic arrow-pushing (箭推机理书写的三大基本规则)
Rules for arrow-pushing:
- Arrows never start with atoms.
- Arrows never start or end on charges.
- Arrows begin with lone pairs, π bonds, or σ bonds, and end on unfilled orbitals.
箭推机理书写规则:
- 箭头不从原子出发。
- 箭头不从电荷出发,也不结束于某个电荷。
- 箭头起始于孤对电子、π 键或 σ 键,终止于未填充轨道。
2.2 HYDROGEN BONDS AND PROTON TRANSFERS (氢键和质子转移)
2.2.1 Hydrogen bonds involve three atoms (氢键涉及三个原子)
Hydrogen bonds are
- mainly a Coulombic interaction.
- sensitive to the polarity of the environment. (Weak in high dielectric water, otherwise in lipid membrane or interior of protein)
- commonly slightly less than 2 Å.
氢键
- 主要是库仑相互作用。
- 强度对环境极性敏感。(高介电常数的水中氢键很弱,低介电常数的脂膜和蛋白质内部氢键很强)
- 通常略短于 2 Å。
Depicting mechanisms involving hydrogen bonds must
- either omit the arrow-pushing for the proton transfer step.
- or omit the formation of the obvious hydrogen bond.
为了正确描绘有氢键参与的机理
- 要么忽略质子转移中的电子转移。
- 要么忽略氢键。
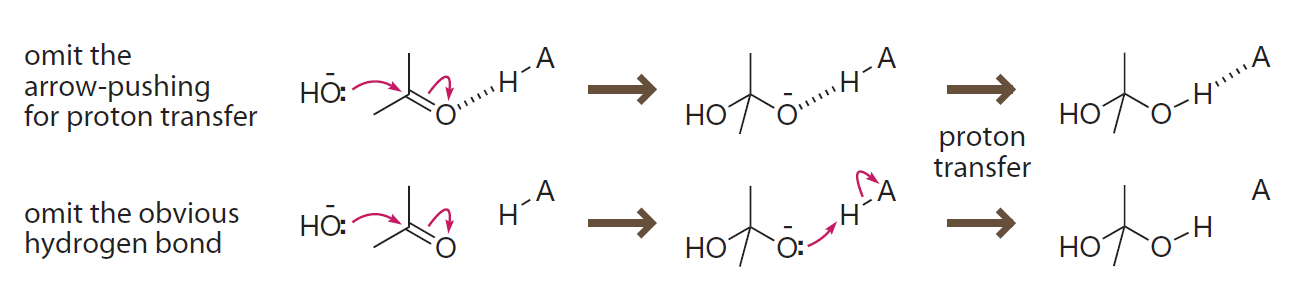
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 34, Figure 2.13
Depicting mechanisms that involves hydrogen bonds
There are two ways to represent a proton transfer that involves a hydrogen bond: omit the curved arrows or omit the hydrogen bond.
2.2.2 Proton transfers to and from heteroatoms are usually very fast (杂原子上的质子转移非常快)
Proton transfers
- are usually very fast to and from oxygen, nitrogen and even sulfur atom. (Except for carbon)
- are actually diffusion-controlled when strongly favorable. (109 M-1 s-1)
- heavily rely on the hydrogen bonds.
质子转移
- 在氧、氮甚至硫上发生的非常快。(除了碳上的质子)
- 较强时,其速率由水溶液扩散控制。(109 M-1 s-1)
- 十分依赖于氢键。
2.2.3 Linear geometries are preferred for proton transfers (线性几何构型是质子转移的最优构型)
Most intramolecular proton transfers
- are actually faster to be done in two steps with the help of protonic acid in medium.
大多数分子内质子转移反应
- 借助环境中的质子酸分两步完成会更加高效。
2.3 PREBIOTIC CHEMISTRY (前生命化学)
2.3.1 HCN and CH2O are key ingredients in the primordial soup (HCN 和 CH2O 是原始汤的主要成分)
Prebiotic chemistry
- attempts to understand and re-create the chemical origin of life.
前生命化学
- 试图解释和重塑生命起源。
Molecular ingredients of life
- could be studied by spectroscopy of interstellar space.
- include water, ammonia, hydrogen cyanide, acetonitrile, acrylonitrile, cyanogen (NC–CN), and cyanoacetylene.
生命的化学原料
- 可以通过宇宙空间的光谱进行研究。
- 包括水、氨气、氰化氢、乙腈、丙烯腈、氰(NC-CN)、丙炔腈等。
From these ingredients:
- Ribose is pentamer of formaldehyde.
- Adenine is pentamer of hydrocyanic acid.
基于上述原料:
- 核糖是甲醛的五聚体。
- 腺嘌呤是氢氰酸的五聚体。
In aqueous solution at room temperature, polymerization of hydrocyanic acid forms
- tetramer diaminomaleonitrile (DAMN).
- larger oligomer to hydrolyze into adenine.
在室温下,氢氰酸水溶液可发生聚合,生成
- 四聚体二氨基马来腈(DAMN)。
- 更大的低聚物并水解生成腺嘌呤。
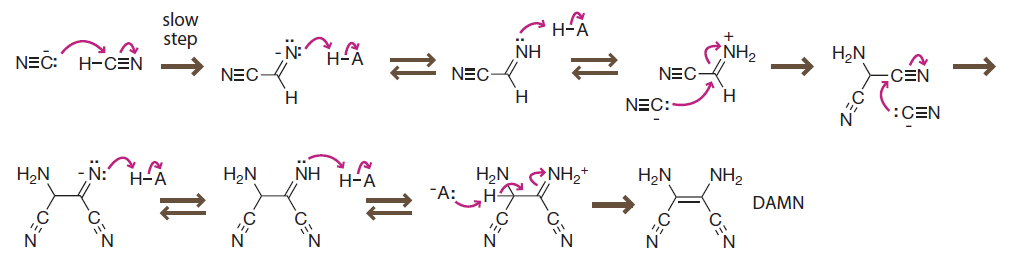
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 37, Figure 2.20
Prebiotic carbon–carbon bond formation
Under basic conditions, cyanide can serve as both a nucleophile and an electrophile, ultimately leading to DNA building blocks like DAMN.
2.3.2 Solutions of HCN contain both nucleophile and electrophile at pH 9.2 (氢氰酸溶液在 pH = 9.2 下存在着亲核体和亲电体)
In a rigorous arrow-pushing mechanism:
- Each set of curved arrows corresponds to a single elementary reaction with one transition state.
- Most steps contain no more than two arrows.
在严格的箭推机理中:
- 每组弯曲箭头对应着一个基元反应,具有一个过渡态。
- 绝大多数的反应步骤不会超过两个箭头。
2.3.3 HCN forms purines and pyrimidines under prebiotic conditions (HCN 在前生命化学条件下生成嘌呤和嘧啶)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
To generate purines:
- Ultraviolet radiation convert DAMN into aminoimidazole carbonitrile (AICN).
- AICN can react with HCN to form adenine, or with urea to form guanine.
关于嘌呤的产生:
- DAMN 在紫外辐射下会生成氨基咪唑腈(AICN)。
- AICN 可以和 HCN 反应生成腺嘌呤,或是和尿素反应生成鸟嘌呤。
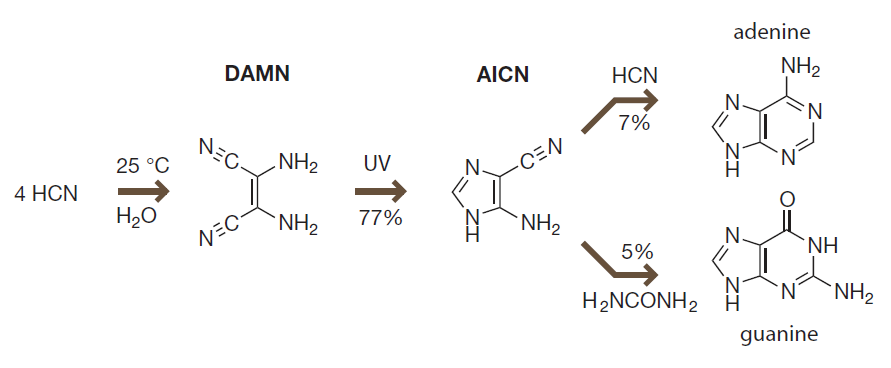
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 38, Figure 2.21
DAMN is a key intermediate for the formation of adenine and guanine under prebiotic conditions
To generate pyrimidines:
- Cyanoacetylene can react with water to form cyanoacetaldehyde.
- Cyanoacetaldehyde then reacts with guanidine to form cytosine.
- Hydrolysis of cytosine would form uracil.
关于嘧啶的产生:
- 丙炔腈与水反应可以生成氰基乙醛。
- 氰基乙醛可以和胍缩合得到胞嘧啶。
- 胞嘧啶进一步可以水解生成尿嘧啶。
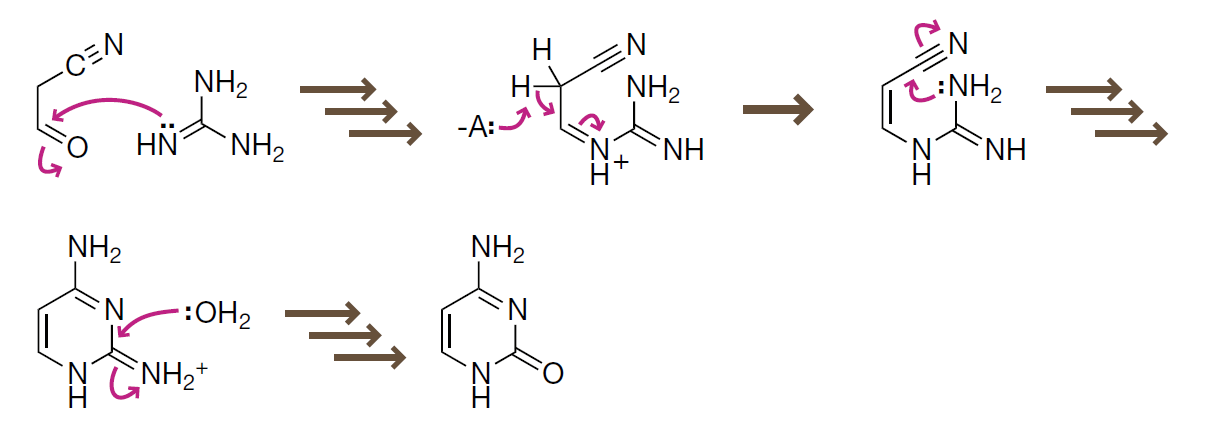
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 39, Figure 2.24
An abbreviated mechanism for formation of cytosine
To save space, we depict only the curved arrows for the first of several mechanistic steps.
When writing arrow-pushing mechanisms,
- omiting some obvious steps by stacking elementary reaction arrows is acceptable.
- we should always leave out intermediate structures and the corresponding curved arrows. (Three-arrow rule)
在书写箭推机理的时候,
- 可以通过堆叠基元反应箭头省略掉一些显而易见的步骤。
- 如果要省略中间体结构,必须同时省略弯曲箭头。(三箭头规则)
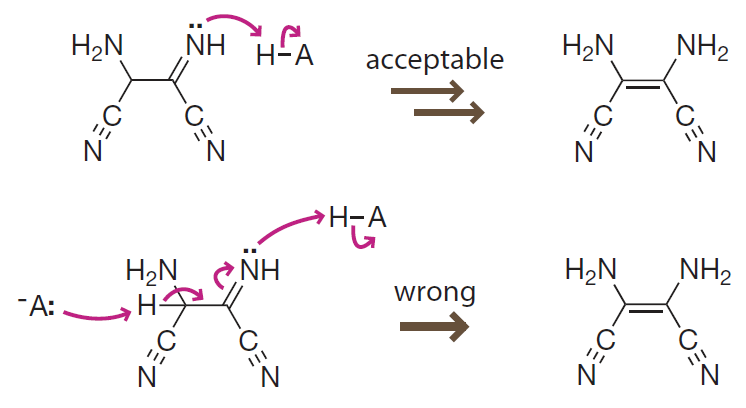
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 39, Figure 2.25
A shortcut for experts
Skipping obvious elementary reaction steps in a mechanism is acceptable as long as the horizontal reaction arrows are retained. However, it is misleading to combine arrows for multiple transition states into a single step.
2.3.4 Aldol reactions with formaldehyde generate carbohydrates (甲醛的羟醛缩合反应可生成碳水化合物)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
Formose reaction
- generates all possible carbohydrates from formaldehyde and glycoaldehyde.
- is actually a series of aldol reaction.
- requires calcium for catalysis.
甲醛聚糖
- 利用甲醛和糖醛生成各种可能的碳水化合物。
- 实际上是一系列的羟醛缩合反应。
- 需要钙离子催化。
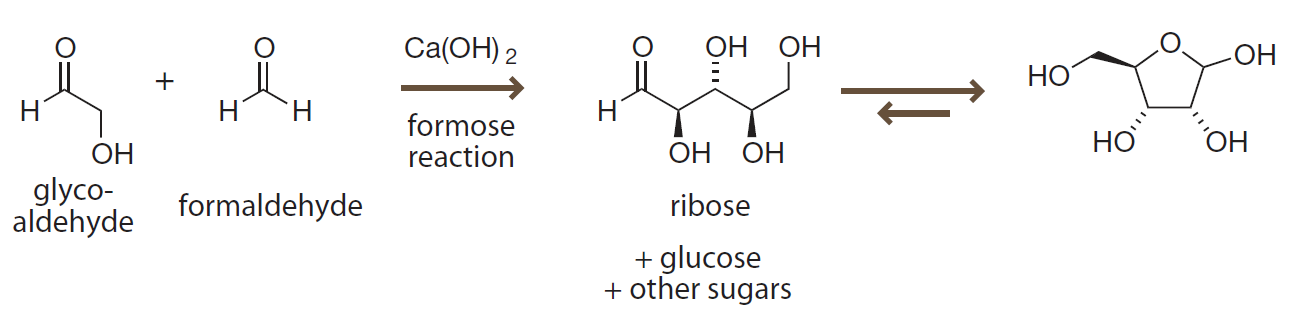
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 40, Figure 2.26
Prebiotic formation of carbohydrates
The formose reaction generates carbohydrates like ribose and glucose under prebiotic conditions.
2.3.5 Cyanide catalyzes the benzoin reaction (氰化物催化安息香缩合反应)
Glycoaldehyde
- was from cyanide-catalyzed benzoin reaction of formaldehyde.
糖醛
- 来源于氰离子催化的甲醛的安息香缩合反应。
2.3.7 Amino acids arise spontaneously under prebiotic conditions (氨基酸在前生命体条件下自发生成)
In famous Miller-Urey experiment:
- Mixture of methane, ammonia, hydrogen and water vapor mocked primordial atmosphere.
- Electrical discharge mocked lighting.
- Several amino acids were found as products.
- Classical Strecker reaction might take place.
在著名的 Miller-Urey 实验中:
- 甲烷、氨气、氢气和水蒸气的混合物被用于模拟原始大气。
- 以放电模拟闪电现象。
- 产生了若干种天然氨基酸。
- 可能发生了著名的 Strecker 反应。
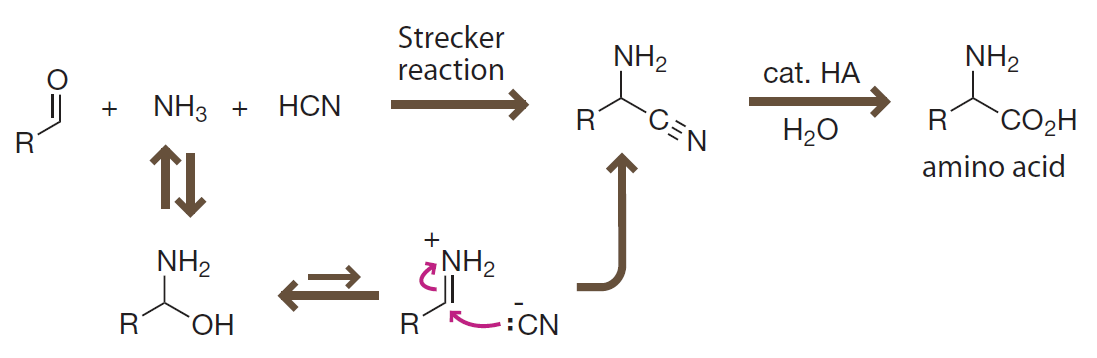
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 42, Figure 2.31
The Strecker reaction
Formation of amino acids under prebiotic conditions involves addition of a cyanide anion to highly reactive iminium ion.
2.4 NONBONDING INTERACTIONS (非键相互作用)
2.4.1 Essentially everything taking place in the cell involves nonbonding interactions (本质上,细胞中发生的一切都涉及非键相互作用)
Nonbonding forces are
- sometimes "strong", regarding the hydrolyzable, thermodynamically weak covalent bonds in biooligomers.
- kinetically labile.
非键相互作用
- 相较于生物分子可水解的共价键在热力学上甚至更加稳定。
- 具有动力学上的易变性。
p.s. Latent translation mistake: the original text says "the covalent bonds that link the subunits of biooligomers are also thermodynamically weak relative to hydrolysis", where "relative" probably means "relevant" rather than "opposite".
注:此处可能有翻译错误,原文说“the covalent bonds that link the subunits of biooligomers are also thermodynamically weak relative to hydrolysis”。这个“relative”我认为指的是“相关的”,而非“相较于”。
2.4.3 For nonbonding interactions, the energies can be fitted to a simplified equation (对于非键相互作用,能量可以转换成一个简化的方程)
Formal charges from Lewis structure
- are often opposite to the actual partial charge.
- could present the net effect of all Coulombic interactions based on partial charges.
Lewis 电子式标注的形式电荷
- 常常和实际的部分电荷相反。
- 可以表达所有部分电荷库仑相互作用的净效应。

Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 44, Figure 2.35
Where is the charge?
The formal charges in Lewis structures offer a misleading picture of the partial charges on the atoms.
2.4.5 It is helpful to distinguish reversible from irreversible interactions (区分可逆与不可逆的相互作用是非常有益的)
Aromatic rings can interact
- with each other either in parallel or in T-shaped structure, giving out roughly equivalent strengths.
- in an offset orientation in order to position regions of electron density over comparatively electron-deficient center of another ring.
- with cations, C-H and the lone pairs of oxygens.
芳环可以
- 以平行或 T 形的方式相互作用,这两种方式的作用强度相近。
- 在作用时发生一定错位以让一个环的富电子区域和另一个环的贫电子中心相互接近。
- 与阳离子、C-H 键和氧原子上的孤对电子相作用。
p.s. For readers interested in π-π stacking interaction, I strongly recommend Angew. Chem. Int. Ed. 2008, 47, 3430–3434 from bigshot in quantum chemistry Prof. Dr Stefan Grimme. Latent translation mistake: the original text says "the arenes prefer an offset or displaced orientation", where "displaced" obviously means "offset" rather than "delocalized".
注:若读者对 π-π 堆叠相互作用感兴趣,我强烈推荐量子化学大牛 Stefan Grimme 教授的 Angew. Chem. Int. Ed. 2008, 47, 3430–3434。同时,此处可能有翻译错误,原文说“the arenes prefer an offset or displaced orientation”。这个“displaced”显然指的是“错位的”,而非“离域的”。
Aqueous environment matters, because
- its high bulk dielectric constant screens charges, destabilizing salt bridge.
- water molecules can form hydrogen bonds to compete with other interactive candidates.
水环境十分重要
- 一方面因为高介电常数的水可以屏蔽电荷,使盐桥作用大幅削弱。
- 另一方面水分子可以形成氢键,和其他物种竞争相互作用。
2.4.6 Entropy makes it difficult to identify favorable states among seemingly endless possibilities (熵使我们很难在看似无穷无尽的可能性中确定有利的状态)
In order to use nonbonding forces to study biological problems without being trapped by effect of entropy,
- we should alway contrast systems with similar configurations.
为了不受熵驱动优势的影响研究涉及非键力的生物问题,
- 我们应当总是比较处于相似构象或构型下的状态。
2.4.7 The hydrophobic effect results from a balance between attractive forces and entropy (疏水效应是由吸引力和熵之间的平衡引起的)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
Comparing hydrogen bonds and dispersive interactions:
- Hydrogen bonds are directional, incurring a high entropic cost.
- Dispersive can easily sum up without a large entropic penalty.
比较氢键和色散作用:
- 氢键是定向的,因此氢键形成往往伴随着很大的熵减。
- 色散作用并没有强烈的取向定向,它们很容易相互叠加而不造成显著的熵减。
2.5 THE POWER OF MODULAR DESIGN (模块化设计的魅力)
2.5.0 Introduction (引子)
Modular design
- reduces the number of molecular parts for structure construction, simplifying the energy-intensive operations.
模块化设计
- 减少了构建细胞结构的分子部件的数量,进而简化了相关的能源密集型操作。
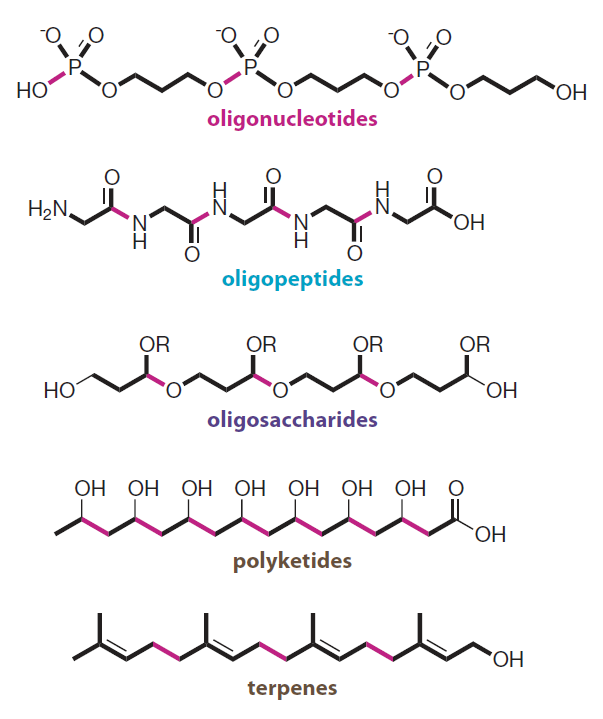
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 49, Figure 2.43
Modular design
The biooligomers shown can be constructed through repetitive bond-forming steps from simple building blocks.
2.5.2 Lability correlates inversely with information longevity (可变性与信息寿命成反比)
p.s. Latent translation mistake: A previously mentioned translation of "lability" exists.
注:此处可能有翻译错误,“lability”在前文中翻译为“可变性”而非“显然更好”。
A molecule with more robust backbone functional groups
- has more longevity and less lability.
- is better as information carrier.
- has less copies in cell.
由更稳定的官能团构成骨架的分子
- 寿命更长,可变性更弱。
- 可更好地作为信息载体。
- 往往在细胞中拷贝更少。
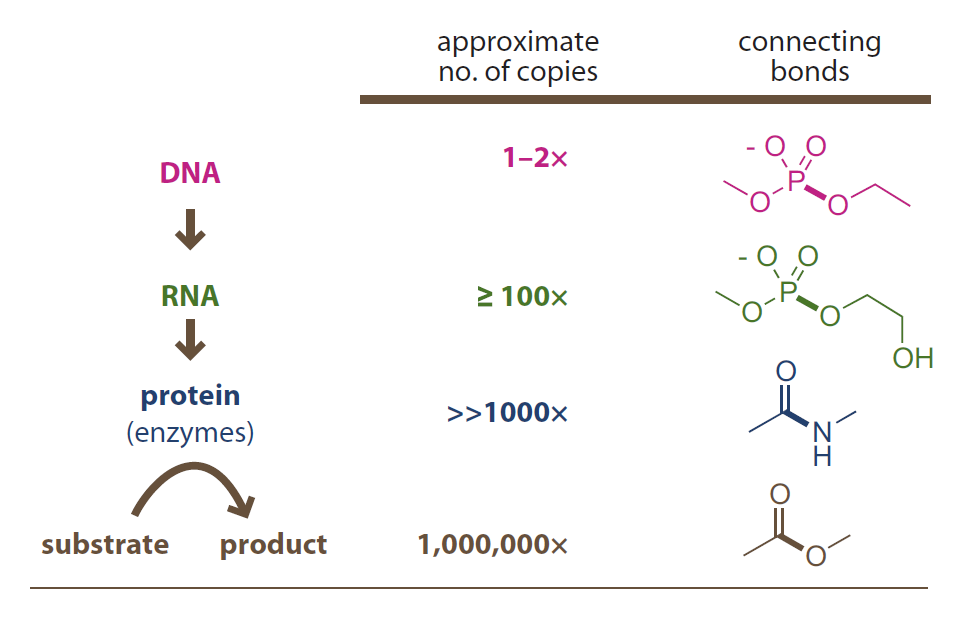
Introduction to Bioorganic Chemistry and Chemical Biology, 2013, Page 49, Figure 2.44
Numerical amplification in biosynthesis
2.5.3 Why are esters more reactive than amides? (为什么酯的反应活性比酰胺强?)
Nitrogen lone pairs are more nucleophilic than oxygen lone pairs,
- making it more likely to donate into carbonyl through resonance in amides.
氮的孤对电子较氧上的亲核性更强,
- 因此孤对电子通过共振作用对羰基的贡献在酰胺中更容易发生。
2.5.4 Why are phosphate esters less reactive than carboxylic esters? (为什么磷酸酯的反应活性不如羧酸酯?)
p.s. Here, the content structure is personally reorganized for conciseness.
注:出于简明考虑,本小节的内容结构有所调整。
For phosphate esters,
- 3p orbital of phosphorus is unlikely to effectively overlap with 2p of oxygen.
- four oxygen with high negative charge on phosphorus tend to repel nucleophiles. (That's why Mg2+ is necessary for phosphodiester hydrolysis)
对于磷酸酯
- 磷的 3p 轨道无法和氧原子的 2p 轨道高效重叠。
- 四个带负电荷的磷原子会强排斥亲核试剂。(所以水解磷酸二酯的酶总是镁离子依赖的)
In the case of pyramidal molecule inversion:
- sp3 center atom undergoes sp2 transition state.
- inverting atom takes s character from axial lone pair for equatorial bonds.
- equatorial electronegative substituents might inhibit such process.
在三角锥分子翻转的情景中:
- 中心原子经历了 sp3 => sp2 的过渡态转变。
- 中心原子从轴向孤对电子那里获取 s 成分并将其应用于平伏键。
- 若平伏键有强电负性取代基,翻转过程将被抑制。
p.s. The author also emphasize the unfavorable contribution of oxygens to the transition state of phosphate ester. It is, however, obviously a minor factor regarding the strong screening effect of negative charged oxygens. And I choose not to underscore it as the author did here. Readers could resort to the original text for more information.
注:此处作者在原文中也强调了磷酸酯上氧原子作为“平伏键强电负性取代基”对过渡态的影响。但我认为这相较于负电荷氧原子对亲核试剂的直接屏蔽显然是次要因素,因此也未多在此论述。感兴趣的读者可自行参阅原文。
Acknowledgements (致谢)
I sincerely thanks Prof. Dr Shi Kuang from Hunan University for her generous help and contribution to the section vide infra:
- Section 2.5.4
M. Zheng-Bing Gao from Wuhan No.3 Middle school for her generous help and contribution to the section vide infra:
- Section 1.0.1
And I thanks M. Zhi-Ming Xing from Hunan University for his contribution to the section vide infra:
- Section 2.5.4
我由衷感谢湖南大学旷实教授对以下小节的慷慨帮助:
- 2.5.4 小节
武汉三中高正兵老师对以下条目的慷慨帮助:
- 1.0.1 小节
此外,我感谢湖南大学刑致明先生对以下小节的贡献:
- 2.5.4 小节
Portal to Catalog of More Notes (链接:学科学习笔记目录)
Portal: here (点左侧链接直达页面)
by Syl & Sylvia @ 2023-06-26 00:06:15 @ Changsha, Yuelu District
originally post @ https://www.cnblogs.com @ 2023-06-26