
The development of various human diseases is highly complex. For drug developers to study human pathogenesis and pathological changes and observe and evaluate the efficacy of drugs, it is necessary to construct models that mimic human diseases, such as organoid or animal models, to explore the nature of human diseases and evaluate the effectiveness of new drugs.
In vitro, ex vivo, or rodent models are generally used in the early stage of drug discovery. As new drug candidates progress from discovery to preclinical development and scale-up drug preparation, large animal models such as Minipigs, dogs, and monkeys are gradually adopted.
The porcine model is one of the large animal models used in many laboratories and drug research sites. The size and characteristics of pigs vary by species. Minipigs were first used in medical research in Europe before being introduced to the United States in the 1980s.
MiniPigs, commonly used as an animal model in pharmaceutical research, are also utilized for both MiniPig Toxicology and Pharmacokinetics studies. Minipigs are similar to humans in size, anatomy, physiology, biochemistry, and genetics. They have become a good model for studying human cardiovascular, respiratory, and skin diseases. Bama Minipigare is usually used as a laboratory pig for human medical research. Bama Minipigare is generally used in human medical research as laboratory pigs.
Minipigs have been used as a standard animal model for drug development for the following reasons.
1. Minipigs are suitable as an animal model for human CYP3A enzyme and its related drug metabolism studies
The porcine model is considered a translational model in biomedical research and development. The physiological and metabolic characteristics of the Bama Minipig are more similar to those of humans, making it more predictable and reliable for use in drug studies to investigate drug metabolism and mechanisms of action.
2. Liver cytochrome P450 enzyme lines (CYPs) are humans' and animals' primary drug-metabolizing enzyme lines.

Cytochrome p450 liver enzyme (CYP3A4, human), chemical structure. This liver protein plays an important role in drug detoxification in the human body. Image Credit: molekuul_be / Shutterstock [1].
Comparing the similarity of pig and human cytochrome P450 enzyme lineage characteristics will be useful to assess the suitability of pigs as an animal model for drug metabolism kinetics.
In the paper "Comparative study of experimental Minipig and human cytochrome P450 enzyme activities", the researchers selected six selective inhibitors of human cytochrome P450 enzymes to test the inhibition of six probe reactions of Minipig cytochrome P450 enzymes.
The results of the activity assay and the inhibition assay not only demonstrated the suitability of the Minipig as an animal model for the study of human CYP3A enzymes and their related drug metabolism, allowing the experimental results to be validated against each other but also demonstrated the scientific validity of using the combined model of the enzyme reaction assay and its selective inhibition assay in the comparison between Minipigs and human cytochrome P450 enzymes.
3. The porcine model is a suitable animal model for ocular research
With the growing popularity of Minipigs in ocular research and drug development, numerous recent Minipig publications are helpful for pathologists and toxicologists involved in ocular toxicity studies.
Gottingen, Yucatan, Hanford, and Sinclair Minipigs have been used in research settings, and the smallest of these Minipig models is the Gottingen, which has rapidly gained popularity among researchers for ocular studies, including those investigating ocular diseases, novel therapeutics, surgical techniques, and implantable materials/devices.
Many animal models have now been developed for use in drug research. Keeping pace with industrial development and market demand, Medicilon's Pharmacology and Pharmacodynamics Department has years of experience and established a complete animal model library based on verifications and practices for precise and efficient drug efficacy testing. The test subjects include non-human primates, dogs, rats/mice, rabbits, guinea pigs, and miniature pigs.
For example, in ophthalmic drug development, Medicilon's preclinical ophthalmic research platform is equipped with an advanced ophthalmic surgical microscope, which enables unique and fine drug delivery to animal species such as rabbits, dogs, Minipigs, and non-human primates, in addition to conventional eye drops and ophthalmic ointment administration.

4. Pig skin is very similar to humans in morphology, physiology, and pharmacology.
Due to the limited source of human tissues, animal models are often used to replace human skin. Among experimental animals, the skin of mice, rats, guinea pigs, rabbits, dogs, and monkeys differ significantly from that of humans. In contrast, pig skin is very similar to human skin regarding morphology and immune response.
The differences between the structural properties of Bama Minipigs and human skin were investigated by gross, light microscopic, and immunohistochemical comparisons, and it was found that the skin layers, thickness, dermal and epidermal connections, distribution of cells in the dermis and epidermis, and arrangement of the extracellular matrix of Bama Minipigs were similar to human skin and suitable as an animal model for drug penetration.
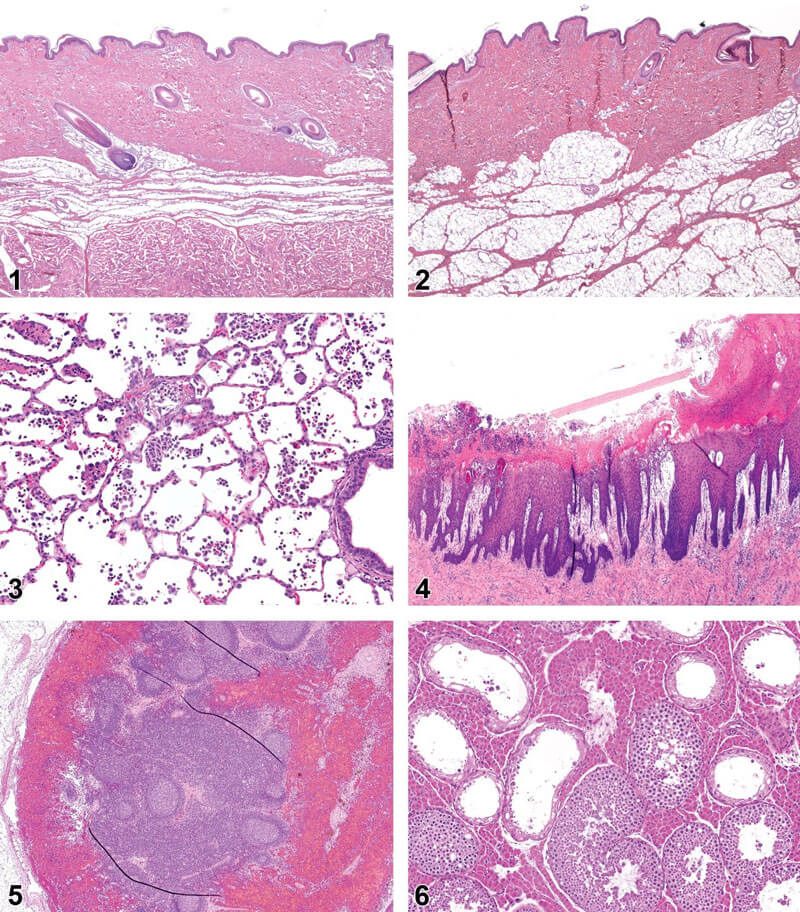
Figure 1. Skin, flank; normal histology. Hematoxylin and eosin. Figure 2.—Skin, caudal to ear; normal histology. Hematoxylin and eosin. Figure 3.—Lung, alveoli; inflammation, subacute/chronic. Hematoxylin and eosin. Figure 4.—Stomach, nonglandular; erosion. Hematoxylin and eosin. Figure 5.—Lymph node; sinus erythrocytosis. Hematoxylin and eosin. Figure 6.—Testicle; tubular degeneration and atrophy. Hematoxylin and eosin.【2】
The skin of pigs is morphologically similar to human skin; however, there are anatomical and physiological differences, and there may be differences between anatomical locations. In pigs, the skin on the flanks (Figure 1) and behind the ears (Figure 2) is the thinnest and most similar to human skin.
5. Development of a small pig animal model for ARS is essential for understanding radiation-induced damage to various organs and tissues
The pig is an important domestic animal and one of the principal model organisms for human biomedical research. e.g., Minipigs can also be used for acute radiation syndrome (ARS) and delayed effects of acute radiation.
For example, small pigs can also be used as animal models of acute radiation syndrome (ARS) and delayed effects of acute radiation exposure (DEARE), which is essential for understanding radiation-induced damage to various organs and tissues.
6. Pig models can evaluate drug safety and efficacy
Because of their smaller size and lower pain perception, Minipigs can better mimic drug responses in humans and other mammals, and thus better assess drug safety and efficacy. Compared to mouse and rat animal models, the Bama Minipig has a longer lifespan than mice and rats and can better mimic the effects of long-term drugs effects.
The following are a few ways the Minipig has been used for drug development.
1. Pharmacokinetics studies
Minipigs are often used in pharmacokinetic studies, which focus on how drugs are absorbed, distributed, metabolized, and excreted in the body. Because Minipigs have anatomy and physiology similar to that of humans, they can provide helpful information about the action of drugs in humans.
To understand the effect of Minipigs on the body's response to drugs, some researchers have developed drug metabolism models of Minipigs. For example, some researchers have conducted pharmacokinetic studies on lovastatin using Minipigs as an animal model, including in vivo studies of single-dose and multi-dose gavage administration, absolute bioavailability studies, and effects on cytochrome P450 enzymes in vitro. Minipigs are more suitable than rats as a good model for drug studies related to the metabolism of human CYP3A enzymes.
Minipigs are very similar to humans in cardiac and circulatory systems, and their serum LDH isoenzyme activity sequence is also identical to that of humans, with LDH1>LDH2, which is called LDH1 derangement, predisposing them to cardiovascular diseases, making Minipigs an ideal model for replicating human cardiovascular diseases. Some investigators have successfully established atherosclerotic stenosis models using Minipigs, which provide an effective pharmacodynamic model for preclinical studies of cardiovascular drugs.
The article "Pharmacokinetics study of gestrinone capsules in Bama Minipigs [3]", To establish a rapid and sensitive HPLC-MS /MS method for determining the concentration of gestrinone in Bama Minipig plasma and to study the pharmacokinetic characteristics of gestrinone Capsules in Bama Minipigs.
Methods: Bama Minipigs were used as experimental animals for 12 weeks, and twice a week, the blood samples were collected at different time points after the administration. The pharmacokinetic parameters were calculated by measuring the concentration of gestrinone in the plasma of Minipigs after multiple administrations. The plasma samples were extracted by cyclohexane and analyzed by LC-MS / MS.
The method is accurate, rapid, sensitive, and specific and is suitable for animal experiments and clinical determination of plasma concentration of gestrinone and its pharmacokinetic study. After repeated administration of gestrinone capsules, there was no difference in 2 h plasma concentration and no adverse reaction after treatment, and the safety was higher.
2.Toxicology studies
Minipigs are also used in toxicology studies, which are studies that examine the safety of drugs. These studies can help researchers determine the potential side effects or adverse reactions that a medicine may cause.
Because of the similarities to human physiology, the pharmaceutical industry places great importance on Minipigs as a non-rodent model for toxicity studies.
The Minipig is currently used in non-clinical pharmacology and preclinical studies for single dose, repeat dose, teratology, fertility assessment, absorption, distribution, metabolism, and excretion studies. Minipig pigs are suitable for a variety of delivery methods, including inhalation, oral intubation, diet, dermal, multiple parenteral routes, and continuous intravenous infusion.
In toxicology studies, the most commonly used non-rodent is the Beagle. Dogs are susceptible to NSAIDs, often causing gastrointestinal damage, erosions, or ulcers at doses below human therapeutic doses, thus limiting the amount administered and masking other side effects. Minipigs are more resistant to gastrointestinal side effects and can successfully complete toxicity experiments with such drugs.
Small pig skin as a source of tissues, organs, and cell cultures can be used instead of human cells for cytotoxicity evaluation of chemicals.
The acute and delayed skin irritation of anthraquinone and its 102 acyl analogs were observed in mice, guinea pigs, and Minipigs, and the time to maximum rage was 24 h in the ear of mice, 48 h in the back of guinea pigs and one week in the back of miniature pigs.
There is a growing trend to use Minipigs in ocular toxicology studies because of their anatomical similarity to the human eye and as a substitute for non-human primates.
Ocular toxicity testing is an essential tool for assessing the safety of new drugs. Animal testing was widely used in early toxicity assessments. However, animal testing has many limitations such as high costs, complex experimental procedures, ethical and legal issues, etc. In recent years, many alternative methods have been proposed, including the use of porcine eyes for ocular toxicity testing. The pig eye is structurally and physiologically similar to the human eye and, therefore, can be used as a substitute for the human eye.
The use of pig eyes for ocular toxicity testing has been extensively studied. Studies have shown that pig eyes can be used to assess the toxicity of various ophthalmic drugs, including antibiotics, antiviral drugs, steroids, NSAIDs, and anticancer drugs.
In addition, several studies have explored the use of porcine eyes for topical irritation and irritant gas testing. These studies suggest that using porcine eyes for ocular toxicity testing is feasible and may provide a valuable alternative to animal testing.
3. Formulation development
Minipigs are commonly used in preclinical studies to evaluate new drug delivery methods and determine how to deliver a drug to the body most effectively.
Mini pigs are particularly well suited for studying drug delivery methods that require local delivery, such as inhalation, intranasal or transdermal routes. They can also evaluate the efficacy of sustained-release formulations or targeted drug delivery systems. So, the use of mini pigs in drug delivery studies can help improve drug development and ultimately benefit human health.
Overall, Bama Minipigs play an essential role in drug development by providing researchers with a valuable animal model that closely mimics human anatomy and physiology.
Shortcomings
Despite the consensus on the superiority of Minipigs as human models, they have not been used much in experiments and are far from taking the place of dogs in toxicology studies.
On the one hand, Beagle dogs have a long history of use in toxicology research, are genetically stable, and have a rich background, and on the other hand, there are limitations in the use of Minipigs.
First of all, it is inconvenient for small pigs to perform experiments. Small pigs are less cooperative than Beagle dogs in experiments, and the experimenter is more physically exhausted during routine operations such as drug administration and blood collection. In addition, the subcutaneous fat of small pigs is thicker, which brings inconvenience to dissection and material collection.
For pharmacological and toxicological studies. The increase in body weight means that the number of drugs administered in the experiment should be increased. For expensive drugs, the experimental costs will be significantly higher in long-term toxicity tests.
For example, the Bama Minipig has shortcomings compared to rodent mice and rats.
High cost: Compared to mice and rats, Bama Minipigs are more costly because they require more feed and more housing space.
Long reproductive cycle: Bama Minipigs have a long reproductive cycle, so obtaining sufficient numbers of experimental animals for long-term studies takes longer.
Difficult to obtain: Compared to mice and rats, the supply of Bama Minipig could be higher, making it more expensive and easier to get experimental animals.
References:
[1] What are Cytochrome P450 Enzymes?
[2] Pigs in Toxicology: Breed Differences in Metabolism and Background Findings
[3] Pharmacokinetics study of gestrinone capsules in Bama Minipigs. GUO Xiang-Jie, LI Zhao, XIE Shu-wu, ZHOU Jie-yun, LI Guo-ting, ZHONG Rui-hua, ZHU Yan